A semi-synthetic plastic used as the base material for motion picture film.
Film Explorer

Sandow the Strong Man (1894). Edison Kinetoscope film on Blair stock.
Film Frame Collection, Seaver Center for Natural History Research, Natural History Museum of Los Angeles County, Los Angeles, CA, United States.

Joueurs de cartes arrosés (1897). Lumière Cinématographe film on cellulose nitrate film stock, from Société des Celluloses Planchon.
Cinematheque française, Paris, France.

Unidentified theater snipe. DuPont cellulose nitrate print film, with tinted base, c. 1937.
Courtesy of Jack Theakston.

King of Jazz (1930). A Two-color Technicolor dye-transfer print, on Eastman Kodak cellulose nitrate film stock.
George Eastman Museum, Rochester, NY, United States.

35mm cellulose nitrate Technicolor print of This is Colour (1942). Nitrate film stock manufactured by Kodak Ltd. in the United Kingdom featured an explosion symbol edge marking in addition to the words NITRATE FILM.
BFI National Archive, Berkhamsted, United Kingdom.
Identification
Eastman Kodak, 1925–1951: “Nitrate Film”; Kodak Ltd. c. 1920–1951: explosion symbol, c. 1930–1951: “Nitrate Film” plus explosion symbol; DuPont: “Nitrate”; Soviet Union: “pozitiv nitro / nitrate positive” (one example).
(Most film manufacturers did not use edge markings to indicate whether a stock was nitrate, but were more likely to indicate “safety” on their acetate stocks.) Eastman Kodak, 1925-1951: “Nitrate Film”; Kodak Ltd. c. 1920–1951: explosion symbol, c. 1930–1951: “Nitrate Film” plus explosion symbol; DuPont: “Nitrate”.
History
Cellulose nitrate was a semi-synthetic plastic used as the base material for motion picture film stock. One of the first plastics, it is highly flammable and chemically unstable, but its transparency, flexibility, and strength made it ideal for the new film medium. Nitrate film remained the global industry standard until the 1950s, when it was replaced by a “high-acetyl” cellulose acetate stock, commonly referred to as cellulose triacetate.
In 1846, German-Swiss chemist Christian Friedrich Schönbein combined cotton fibers with nitric and sulfuric acids, creating cellulose nitrate. The explosive material had obvious military applications (guncotton), but with chemical alterations also proved useful for other industries (del Amo, 2000: pp. 42–44). Key to the burgeoning field of photography were experiments with pyroxylin, a refined, non-explosive form of cellulose nitrate, with a lower nitrogen content. Dissolving pyroxylin in ethyl alcohol produced a colorless, viscous liquid called collodion, which was quickly adopted by photographers to adhere photosensitive emulsion to glass plates (Rossell, 2002: p. 38). Combining pyroxylin with solvents and plasticizers, particularly camphor, produced a transparent solid material that could be molded into shapes using heat, or pressure, and colored with dyes, fillers, or paints. First patented by English engineer Alexander Parkes in 1865 and sold under a variety of trade names, this new plastic was commonly called “celluloid”, thanks to Americans John Wesley Hyatt and Isiah Smith Hyatt. In 1872, the Hyatt brothers patented a superior manufacturing process and the following year, they relocated their business, the newly renamed Celluloid Manufacturing Company, from Albany, New York, to Newark, New Jersey, and soon dominated the market for pyroxylin-based plastics (Kaufman, 1963: pp. 17–40; Mossman, 1994: pp. 11–22).
Despite its well-known flammability, celluloid was used to make a range of products, including billiard balls, dentures, combs, shirt collars, toys, and decorative objects (Rossell, 2002: pp. 39-40). The material’s strength and transparency also made it suitable as a photographic base (Friedel, 1983: p. 91). In 1888, John Carbutt’s Keystone Manufacturing Company in Philadelphia, Pennsylvania, became the first supplier of celluloid photographic plates – manufactured using celluloid supplied by the Celluloid Manufacturing Company (Rossell, 2002: p. 41; Spehr, 2000: p. 5). Using a solvent called amyl acetate, patented by the Celluloid Manufacturing Company, Henry Reichenbach, chemist at the Eastman Dry Plate Company (later Eastman Kodak) in Rochester, New York, further refined the celluloid mixture to create a thin, flexible plastic base for Eastman’s new photographic roll film. Reichenbach filed a patent for his celluloid roll film in April 1889. (Friedel, 1983: p. 93; Rossell, 2002: pp. 42–3; Spehr, 2000: p. 6). In 1887, Reverend Hannibal Goodwin of Newark, New Jersey, had filed a similar patent, but issues with the amateur inventor’s descriptions of his processes delayed its approval until 1898. In 1914, after many years of courtroom battles, Eastman Kodak and a few other film manufacturers were found to have infringed Goodwin’s patent, then held by photographic wholesalers Anthony and Scoville (later Ansco). By this time, however, the patent had nearly expired and Eastman’s roll film business was firmly established (Friedel, 1983: pp. 93–4; Rossell, 2002: p. 50, n. 37). Eastman began manufacturing celluloid roll film in February 1890 (Rossell, 2022: p. 33). Their chief competitor, the Blair Camera Company in Boston, MA, began commercial production of their own photographic roll film in late 1891, using celluloid from the Celluloid Manufacturing Company (Spehr, 2000: pp. 3–28; Rossell, 2002: pp. 41–4).
Celluloid roll film immediately proved useful for experiments in scientific and moving-image photography. Étienne-Jules Marey, for example, used Eastman celluloid roll film for demonstrations of his sequence camera in Paris, November 1890 (Rossell, 2022: p. 37). In 1899, W. K. L. Dickson also turned to Eastman for help in adapting celluloid roll film for the motion picture equipment being developed at Thomas Edison’s laboratory. The Kinetograph camera and the Kinetoscope viewer required narrower, thicker celluloid, able to withstand the camera’s stop-start movement as well as repeated viewings. After a great deal of experimentation, Dickson and Eastman settled on a filmstrip 35mm wide, which would evolve into the standard gauge for professional filmmaking (Spehr, 2000: pp. 7–21). In 1892, Eastman suspended roll film production due to personnel issues, and so Dickson ordered customized 35mm stock from the Blair Camera Company, to shoot the first Kinetoscope films, such as Blacksmith Scene (1893) and Sandow the Strong Man (1894) (Spehr, 2000: pp. 13–14).
At the same time, the European Blair Camera Company, based in England, was supplying many early filmmakers overseas, including Robert Paul in the United Kingdom, the Lumière Brothers in France, and Oskar Messter in Germany. European Blair was founded in 1893 after a corporate restructuring, and though technically a separate company, it retained access to the patents and manufacturing methods of its American counterpart. Eastman resumed roll film production in early 1894, initially restricting moving image film to special custom orders. In 1896, in response to growing demand in America and Europe and increasing competition from Blair, Eastman began regular production of 35mm motion picture film, regaining Edison’s significant business (Spehr, 2000: pp. 18–19). Eastman Kodak then purchased American Blair in 1899, securing the patents for the company’s superior manufacturing methods, and becoming the largest producer of celluloid film in the United States (Rossell, 2002: pp. 43–5). Eastman also supplied nitrate film to many other parts of the world, with subsidiaries in Canada, England, France and Germany (Layton, 2020: pp. 240–7).
Celluloid was both dangerous and difficult to manufacture, and suppliers were limited, particularly in the early decades of the film industry. A number of film stock companies entered the market, only to fold after a few years, while others, like Blair, purchased the celluloid base from an outside manufacturer and then coated it with proprietary emulsions (Rossell, 2002: pp. 44–5; Rossell, 2024). France first began nitrate film production in 1896, when the Lumière Brothers joined with sheet film manufacturer Victor Planchon, to found the Société des Celluloses Planchon in Lyon (Spehr, 2000: p. 17; Rossell, 2002: p. 45). Pathé Frères purchased European Blair in 1907, and manufactured its own stock until a merger with Eastman Kodak in 1931 (Salt, 1992: p. 63; Blot-Wellens, 2020: pp. 206–7). In Germany, Agfa (Aktien-Gesellschaft für Anilinfabrikation) began manufacturing moving image film around 1899, and merged with the American company Ansco in 1928. Ansco became independent again after World War II (Berriatúa & Blot-Wellens, 2020: pp. 257–8; Pritchard, 2020: p. 307). Gevaert, in Belgium, appears to have started manufacturing moving image film around 1910; while Ferrania in Italy, started in the mid-1920s; and the British photographic plate company Ilford launched its first film stock, Selo, in 1923 (Blot-Wellens, 2020, pp. 267–8; Pritchard, 2020: pp. 308–10). Efforts towards initiating domestic film stock production in the Soviet Union, began in 1926, initially with help from the French industry. The first factory in Pereslavl-Zalessky, Russia, began operating in 1931. Three others, located in Shostka, Ukraine; Kazan, Russia; and Leningrad, Russia, had opened by 1935. By 1937, the USSR claimed to be the third largest film stock manufacturer in the world (Bagrov, 2020: pp. 286–9). Eastman Kodak’s primary domestic competition after Blair, was DuPont, a chemical manufacturing company looking to diversify its operations after World War I. DuPont began production of motion picture film in 1926, initially in partnership with the distributor Pathé Exchange, and while it is unclear exactly how much of the market share the company claimed, DuPont briefly challenged Eastman’s dominance and spurred a period of rapid innovation in film emulsions (Marzola, 2015: pp. 7–11). The last major nitrate film manufacturer to enter the market was Fuji in Japan, which began production in 1934 (Okada, 2020: p. 283).
Nitrate film ignites easily and burns quickly. It is difficult to extinguish and releases hazardous gasses. Early cinema-related fires – such as those at the Bazar de la Charité Fair in Paris, in May 1897; at a traveling bioscope show in Bilston, Staffordshire, England, in July 1898; and at Salão de Novidades Paris no Rio, the first movie theater in Rio de Janeiro, Brazil, in August 1898 – led to regulations governing the handling, shipment and exhibition of nitrate film. Restrictions and safety measures were especially stringent for film exchanges, where film was stored in large quantities, and theater projection booths, where risk to human life was considered higher (McQueen, 2018: pp. 107–10). Given the risks and regulations, experiments began almost immediately to replace cellulose nitrate with a non-flammable plastic. Cellulose acetate – created by combining cotton cellulose fibers with plasticizers and acetyl acid – was the most viable alternative, and from the 1910s through the 1930s, several different chemical formulas were produced, including cellulose diacetate, cellulose acetate butyrate, and cellulose acetate propionate. Industry personnel, however, consistently found these early acetate stocks to be inferior to nitrate, particularly in terms of tensile strength and toughness. They were also more expensive to manufacture (Smither & Surowiec, 2002: pp. 319–25). So, while acetate stock in a variety of gauges was available from the 1910s onward for amateur filmmaking, non-theatrical exhibitions, and special circumstances like military filmmaking, nitrate remained the professional standard for 35mm theatrical film exhibition until after World War II (Enticknap, 2002: pp. 203–10).
In 1947, Eastman Kodak released a “high-acetyl” form of cellulose acetate deemed comparable to nitrate on all significant commercial measures, and, in 1950, the company announced that production of nitrate stock had ceased at its Rochester, NY, plant (Fordyce, 1948: pp. 331–50; Enticknap, 2002: pp. 209–10). However, movies continued to be shot on existing nitrate stock, and different countries converted to acetate at differing rates, with the majority phasing-out nitrate between 1950 and 1956. In some countries – including China, the Soviet Union, and Francoist Spain – nitrate film was used for new productions well into the 1960s (FIAF, 1991: p. 20; Bagrov, 2020: p. 287; Peter Bagrov, email to James Layton, May 21, 2024; Arnau & Christensen, 2002: p. 309).
As nitrate film was phased out of the film industry, it became instead the concern of film archives, and by the 1970s, many repositories viewed it as a burden. First, it poses an obvious fire risk, and numerous institutions – including the National Archives in Suitland, MA, United States; the Cineteca Nacional, in Mexico; and the Cinemateca Brasileira, São Paolo, Brazil – have lost valuable cinematic heritage to nitrate film fires. In addition, cellulose nitrate’s inherent chemical instability causes it to decompose over time. Believing nitrate film would last only 50 years, many archives in the 1970s instituted “copy and destroy” policies, duplicating their nitrate holdings onto acetate and disposing of the originals (Culhane, 1977: pp. 54–5). Unfortunately, cellulose acetate also decomposes over time and ultimately proved less stable than nitrate. Nitrate film will, in fact, last hundreds of years if stored under the proper temperature and relative humidity conditions (Adelstein et al., 2002: pp. 136–43). So, while nitrate film remains subject to safety regulations, including fireproof storage vaults and specialized shipping and disposal, a small number of film heritage institutions, such as the George Eastman Museum in Rochester, New York, have regulation-compliant projection booths that allow original nitrate prints to be screened for twenty-first-century audiences.

An early film can from Dr. J. H. Smith & Co. in Zurich, Switzerland, without any markings to indicate that the film inside was nitrate and flammable.
Cinematheque française, Paris, France.

DuPont 35mm nitrate film can with warning label.
The Museum of Modern Art, New York, NY, United States.

Aftermath of a fire at the Clawson Film Laboratory in Salt Lake City, Utah, as evidence of the hazards posed by nitrate film.
A. J. Snow (1930). “Salt Lake City Film Fire”. Quarterly of the National Fire Protection Association, 23:3 (January): p. 253.
Selected Filmography
Feature film shot on a combination of cellulose nitrate and cellulose acetate stock during a transitionary period, shortly after Eastman Kodak ceased production of nitrate film.
Feature film shot on a combination of cellulose nitrate and cellulose acetate stock during a transitionary period, shortly after Eastman Kodak ceased production of nitrate film.
Short film produced for the first Kinetoscope parlors, shot on cellulose nitrate stock from the Blair Camera Company.
Short film produced for the first Kinetoscope parlors, shot on cellulose nitrate stock from the Blair Camera Company.
Short film shot as an early experiment in motion picture film production on cellulose nitrate stock from Eastman Kodak.
Short film shot as an early experiment in motion picture film production on cellulose nitrate stock from Eastman Kodak.
Feature film shot on cellulose nitrate stock from Ferrania.
Feature film shot on cellulose nitrate stock from Ferrania.
Feature film shot on cellulose nitrate stock from Agfa (Aktien-Gesellschaft für Anilinfabrikation).
Feature film shot on cellulose nitrate stock from Agfa (Aktien-Gesellschaft für Anilinfabrikation).
Short documentary printed on cellulose nitrate stock, which was manufactured in the USSR until the mid-1960s.
Short documentary printed on cellulose nitrate stock, which was manufactured in the USSR until the mid-1960s.
Short film shot with the Cinématographe camera/projector on cellulose nitrate stock from the European Blair Camera Company.
Short film shot with the Cinématographe camera/projector on cellulose nitrate stock from the European Blair Camera Company.
Feature film shot on cellulose nitrate stock. The original negative was lost in a nitrate fire at the Yokohama Cinema Developing Laboratory.
Feature film shot on cellulose nitrate stock. The original negative was lost in a nitrate fire at the Yokohama Cinema Developing Laboratory.
Animated film believed to be printed on cellulose nitrate film stock, which was manufactured in China until the early-1960s.
Animated film believed to be printed on cellulose nitrate film stock, which was manufactured in China until the early-1960s.
Technology
Cellulose nitrate, or celluloid, is a clear, flammable, semi-synthetic polymer. When cellulose cotton fibers are mixed with nitric and sulfuric acids, the sulfuric acid catalyzes a chemical reaction called nitration, where the hydroxyl groups (OH) in the cellulose polymer chain are replaced by nitrate groups (NO2) (del Amo, 2000: pp. 42–4). In the manufacture of motion picture film, plasticizers like camphor and solvents like amyl acetate were added to the nitrated cellulose to make the material more uniform and flexible. This allowed it to be shaped into long, thin strips, onto which photographic emulsion could be applied.
Nitrate film manufacturing was a complex and dangerous business, but by the 1930s, the Eastman Kodak Company in Rochester, New York, had developed an efficient process. First, cotton fibers were bathed in a mixture of nitric acid, sulfuric acid and water, and then rinsed in hot water and butyl alcohol. The fibers were dried in a centrifuge and dissolved in a mixture of the solvents methyl alcohol, ethyl alcohol and acetone. Camphor was added to create a clear dope. Initially, Eastman spread the dope onto large glass tables and cut the dried celluloid into strips, but with inconsistent results. Later, the dope was funneled through a narrow slit onto a large rotating wheel to form strips, a method acquired from the Blair Camera Company, when Eastman purchased it in 1899 (Spehr, 2000: pp. 6–13; Rossell, 2002: pp. 42–3). The celluloid was then dried using hot air, coated with gelatin, and submerged in a bath of melted emulsion. The film was chilled to set the emulsion and dried under careful conditions. Finally, the film was cut to the desired length and perforated, using calibrated dies and punches (Carver, 1937: pp. 596–603).
Due to its chemical composition, nitrate film burns easily and quickly. When ignited, it generates both hazardous gasses and oxygen – which further fuels the fire – making it difficult to extinguish. The structure of the cellulose nitrate polymer is also inherently unstable. Hydrolysis – a reaction in which water molecules break chemical bonds – detaches the nitrate groups from the polymer’s cellulose backbone: this is called denitration. The cellulose polymer chain also breaks down, over time, into smaller units – in a process called chain scission. These chemical processes are autocatalytic, generating byproducts, such as nitric acid, that further catalyze decomposition reactions (Kepley et al., 2015: p. 19).
Denitration and chain scission cause noticeable changes to nitrate film’s physical properties, which are commonly described using a five-stage model, first outlined in A Handbook for Film Archives (FIAF, 1991). Stage 1: the image fades, the celluloid yellows and becomes brittle, and the film begins to emit a noxious odor. Stage 2: the emulsion becomes sticky. Stage 3: the emulsion blisters and bubbles, and the odor becomes stronger. Stage 4: the film congeals into a solid mass, sometimes forming a “viscous froth.” Finally, Stage 5: the film disintegrates into an inert brown powder.
Heat and moisture speed up the decomposition process, and it is hypothesized that variations in manufacturing and processing conditions, including chemicals used in stop baths and fixing solutions, if not properly removed by washing, may also have an effect (Kepley et al., 2015: p. 18). However, if nitrate film is stored at cold temperatures and low relative humidity (RH), decomposition can be slowed, or even halted. Current best practices recommend 2° C (35.6° F) and 23–30 % RH. Storing films in vented cans allows gasses to escape and prevents further decomposition. Under these conditions, nitrate film is estimated to last over 100 years (Bigourdan, 2000: pp. 56–62).
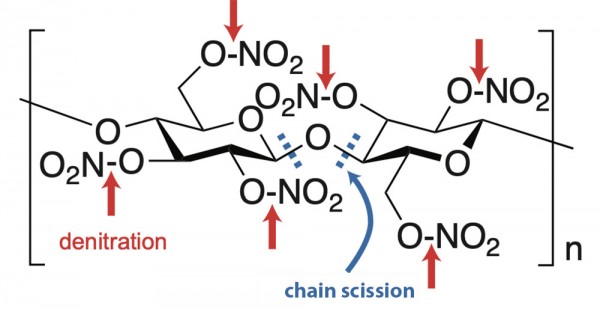
Processes of denitration and chain scission, in the decomposition of cellulose nitrate.
Mahesh Mahanthappa (2015). “Investigation of cellulose nitrate motion picture film chemical decomposition and associated fire risk”. National Endowment for the Humanities (Project ID: PR-50141-12). Wisconsin Center for Film and Theater Research/ University of Wisconsin-Madison, Department of Chemistry/Wisconsin Historical Society.

Diagram by Crystal Kui. Images courtesy of George Eastman Museum and Wisconsin Center for Film and Theater Research.
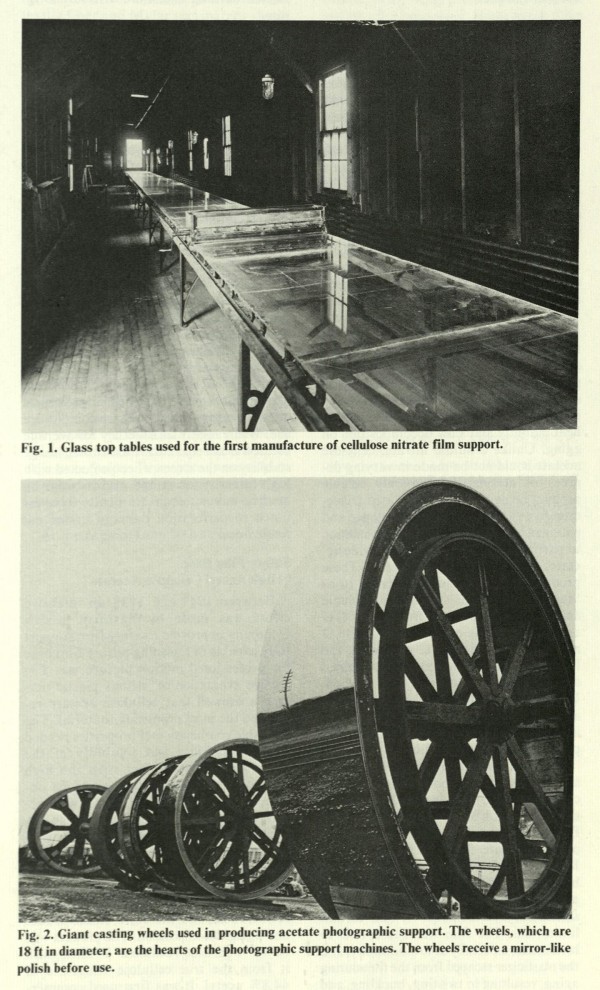
Glass top tables, used to manufacture cellulose nitrate film at Eastman Kodak until 1901. They were replaced by large continuous-casting wheels after Kodak purchased the Blair Camera Company.
Charles R. Fordyce (1976). “Motion Picture Film Support, 1889–1976: An Historical Review”. Journal of the SMPTE Journal 85:7 (July): p. 493.
References
Adelstein, P. Z., J.M. Reilly & F. G. Emmings (2002). “Stability of Ester Base Photographic Film Part VI – Long-term Aging Studies”. Journal of the SMPTE, 111:4 (April): pp. 136–43.
Arnau, Rosa Cardona & Jennifer Gallego Christensen (2002). “Interview with Enrique Blanco, January 2000”, pp. 304–10. In This Film is Dangerous: A Celebration of Nitrate Film, Roger Smither & Catherine A. Surowiec (eds). Brussels: FIAF.
Bagrov, Peter (2020). “Preliminary Notes on Soviet Nitrate Film Stock and Other Aids to Identification of Russian and Soviet Films”, pp. 285–305. In Physical Characteristics of Early Films as Aids to Identification: New Expanded Edition, Camille Blot-Wellens (ed.). Bloomington, IN: Indiana University Press/FIAF.
Berriatúa, Luciano & Camille Blot-Wellens (2020). “Agfa”, pp. 257–66. In Physical Characteristics of Early Films as Aids to Identification: New Expanded Edition, Camille Blot-Wellens (ed.). Bloomington, IN: Indiana University Press/FIAF.
Bigourdan, Jean-Louis (2002). “From the Nitrate Experience to New Film Preservation Strategies”, pp. 52–73. In This Film is Dangerous: A Celebration of Nitrate Film, Roger Smither & Catherine A. Surowiec (eds). Brussels: FIAF.
Blot-Wellens, Camille (2020a). “Gevaert and Agfa-Gevaert”, pp. 194–228. In Physical Characteristics of Early Films as Aids to Identification: New Expanded Edition, Camille Blot-Wellens (ed.). Bloomington, IN: Indiana University Press/FIAF.
Blot-Wellens, Camille (2020b). “Pathé”, pp. 267–76. In Physical Characteristics of Early Films as Aids to Identification: New Expanded Edition, Camille Blot-Wellens (ed.). Bloomington, IN: Indiana University Press/FIAF.
Carver, E. K. (1937). “The Manufacture of Motion Picture Film”. Journal of the SMPE, 28:6 (June): pp. 594–603.
Culhane, John (1977). “Nitrate Won’t Wait”. American Film, 2: pp. 54–9.
Del Amo, Alfonso (2000). “A Brief History of Plasticized Cellulose Nitrate or Celuloid [sic]”. Journal of Film Preservation. 60/61: pp. 41–5.
Enticknap, Leo (2002). “The Film Industry’s Conversion from Nitrate to Safety Film in the Late 1940s: A Discussion of the Reasons and Consequences”, pp. 203–12. In This Film is Dangerous: A Celebration of Nitrate Film, Roger Smither & Catherine A. Surowiec (eds). Brussels: FIAF.
FIAF (1991). Handling, Storage, and Transport of Cellulose Nitrate Film. Brussels: FIAF/UNESCO.
Fordyce, Charles R. (1948). “Improved Safety Motion Picture Film Support”. Journal of the SMPE, 51:11 (Oct.): pp. 331–50.
García Fernández-Villa, Silvia & Margarita San Andrés Moya (2005). “Original Patents as an Aid to the Study of the History and Composition of Semisynthetic Plastics”. Journal of the American Institute for Conservation, 44:2: pp. 95–102.
Kaufman, M. (1963). The First Century of Plastics: Celluloid and Its Sequel. London: The Plastics and Rubber Institute.
Kepley, Vance, Mahesh Mahanthappa, Kathleen Mullen, Mary Huelsbeck & Amanda McQueen (2015). “Investigation of cellulose nitrate motion picture film chemical decomposition and associated fire risk.” National Endowment for the Humanities (Project ID: PR-50141-12). Wisconsin Center for Film and Theater Research/ University of Wisconsin-Madison, Department of Chemistry/Wisconsin Historical Society.
Layton, James (2020). “Eastman Kodak”, pp. 229–56. In Physical Characteristics of Early Films as Aids to Identification: New Expanded Edition, Camille Blot-Wellens (ed.). Bloomington, IN: Indiana University Press/FIAF.
Marzola, Luci (2015). “Better Pictures Through Chemistry: DuPont and the Fight for the Hollywood Film Stock Market”. The Velvet Light Trap, 76: pp. 3–18.
McQueen, Amanda (2018). “Flammable Workhorse: A History of Nitrate Film from the Screen to the Vault”, pp. 103–17. In Routledge Companion to Media Technology and Obsolescence, Mark Wolf (ed.). London: Routledge.
Mossman, S. T. I. (1994). “Parkesine and Celluloid”. In The Development of Plastics, S. T. I. Mossman & P. J. T. Morris (eds). London: Royal Society of Chemistry.
Okada, Hidenori (2020). “Fuji”, pp. 283–4. In Physical Characteristics of Early Films as Aids to Identification: New Expanded Edition, Camille Blot-Wellens (ed.). Bloomington, IN: Indiana University Press/FIAF.
Okajima, Hisashi & Yoshiro Irie (2002). “Kyoto Tales and Tokyo Stories: Incidents in Japanese Film History”, pp. 482–5. In This Film is Dangerous: A Celebration of Nitrate Film, Roger Smither & Catherine A. Surowiec (eds). Brussels: FIAF.
Pritchard, Brian (2020). “Some Information on Other Film Stock Manufacturers”, pp. 307–13. In Physical Characteristics of Early Films as Aids to Identification: New Expanded Edition, Camille Blot-Wellens (ed.). Bloomington, IN: Indiana University Press/FIAF.
Rossell, Deac (2002). “Exploding Teeth, Unbreakable Sheets, and Continuous Casting: Nitrocellulose, from Guncotton to Early Cinema”, pp. 37–51. In This Film is Dangerous: A Celebration of Nitrate Film, Roger Smither & Catherine A. Surowiec (eds). Brussels: FIAF.
Rossell, Deac (2022). Chronology of the Birth of Cinema 1833–1896. New Barnet, UK & Bloomington, IN: John Libbey Publishing/Indiana University Press.
Rossell, Deac (2024). “Tabelle Cine Raw Stock Production”. Courtesy of Deac Rossell.
Salt, Barry (1992). “Film Style and Technology: 1907–1913”, pp. 62–110. In Film Style and Technology: History and Analysis. (2nd exp. edn). Brentford: Starword.
Smither, Roger & Catherine A. Surowiec (2002a). “A Calendar of Film Fires”, pp. 429–53. In This Film is Dangerous: A Celebration of Nitrate Film, Roger Smither & Catherine A. Surowiec (eds). Brussels: FIAF.
Smither, Roger & Catherine A. Surowiec (2002b). “Safety Film: False Dawns”, pp. 319–27. In This Film is Dangerous: A Celebration of Nitrate Film, Roger Smither & Catherine A. Surowiec (eds). Brussels: FIAF.
Spehr, Paul C. (2000). “Unaltered to Date: Developing 35mm Film”, pp. 3–27. In Moving Images: From Edison to the Webcam, John Fullerton & Astrid Söderbergh Widding (eds). New Barnet: Aura/John Libbey & Co.
Worden, Edward Chauncy (1911). Nitrocellulose Industry: A Compendium of the History, Chemistry, Manufacture, Commercial Applications and Analysis of Nitrates, Acetates and Xanthates of Cellulose as Applied to the Peaceful Arts (Vol. 2). New York: D. Van Nostrand Company.
Patents
Parkes, Alexander. Improvements in the manufacture of parkesine or compound of pyroxyline, and also solutions of pyroxyline known as collodion. UK patent BP1313, issued 1865.
Hyatt, Isaiah Smith and John Wesley Hyatt. Improvement in processes and apparatus for manufacturing pyroxyline. US patent US133229, issued November 19, 1872. https://patents.google.com/patent/US133229A
Stevens, John H. Manufacture of compounds of pyroxyline or nitro-cellulose. US patent US269,340, filed June 12, 1882, and issued December 19, 1882. https://patents.google.com/patent/US269340A
Goodwin, Hannibal Williston, Photographic Pellicule and Process of Producing Same. US patent US610,861, filed May 3, 1887, issued September 13, 1898.
Eastman, George. Improvements in flexible photographic film. US patent US306,284, filed April 9, 1889.
Reichenbach, Harry M. Manufacture of flexible photographic films. US patent US417,202, filed April 9, 1889, and issued December 10, 1889. https://patents.google.com/patent/US417202A
Blair, Thomas Henry and Stukely E. Waterman. Method of and apparatus for making photographic films. US patent US588,790, filed January 3, 1894, and issued August 24, 1897. https://patents.google.com/patent/US588790A
Related entries
Author
Amanda McQueen is a media historian and archivist at the University of Arkansas Little Rock Center for Arkansas History and Culture. She has an MA and PhD in Communication Arts from the University of Wisconsin-Madison and an MA in Moving Image Archiving and Preservation from New York University. She is co-author of “Investigation of Cellulose Nitrate Motion Picture Film Chemical Decomposition and Associated Fire Risk” (2015), the white paper for the Wisconsin Nitrate Film Project, funded by the National Endowment for the Humanities; and author of “Flammable Workhorse: A History of Nitrate Film from the Screen to the Vault” in Routledge Companion to Media Technology and Obsolescence (2019). In addition, McQueen has published on Classical Hollywood stardom, contemporary sound design norms, and the musical on stage and screen. She also works part-time as a cataloger for the Texas Archive of the Moving Image, Austin.
Thanks to Heather Heckman and Vance Kepley.
McQueen, Amanda (2025). “Cellulose nitrate”. In James Layton (ed.), Film Atlas. www.filmatlas.com. Brussels: International Federation of Film Archives / Rochester, NY: George Eastman Museum.